Introduction
Free light chains (FLCs) are a well-established biomarker of plasma cell dyscrasias. FLCs exist as monomers, dimers and oligomers. Lambda FLC is particularly prone to form dimers and oligomers. Under certain pathological conditions, such as in AL amyloidosis and multiple myeloma (MM), high levels of FLC dimers can be found even when total FLC concentrations were near normal levels.
Therefore, quantification of FLC dimers can be used as a potential tool to diagnose and monitor AL amyloidosis and MM. Here we report a novel assay, Dimerite™, to detect and quantify FLC dimers with mass spectrometry. To compare the performance of the Dimerite assay, we quantified FLC dimers in LC-only MM serum samples and compared the results to the gold standard Freelite assay.
Study Summary
FLC dimer quant assay was compared to Freelite assay for 17 newly diagnosed patients with LC-only MM. All patients underwent autologous stem cell transplant (ASCT) and had banked serum samples at diagnosis and day 100 post-ASCT.
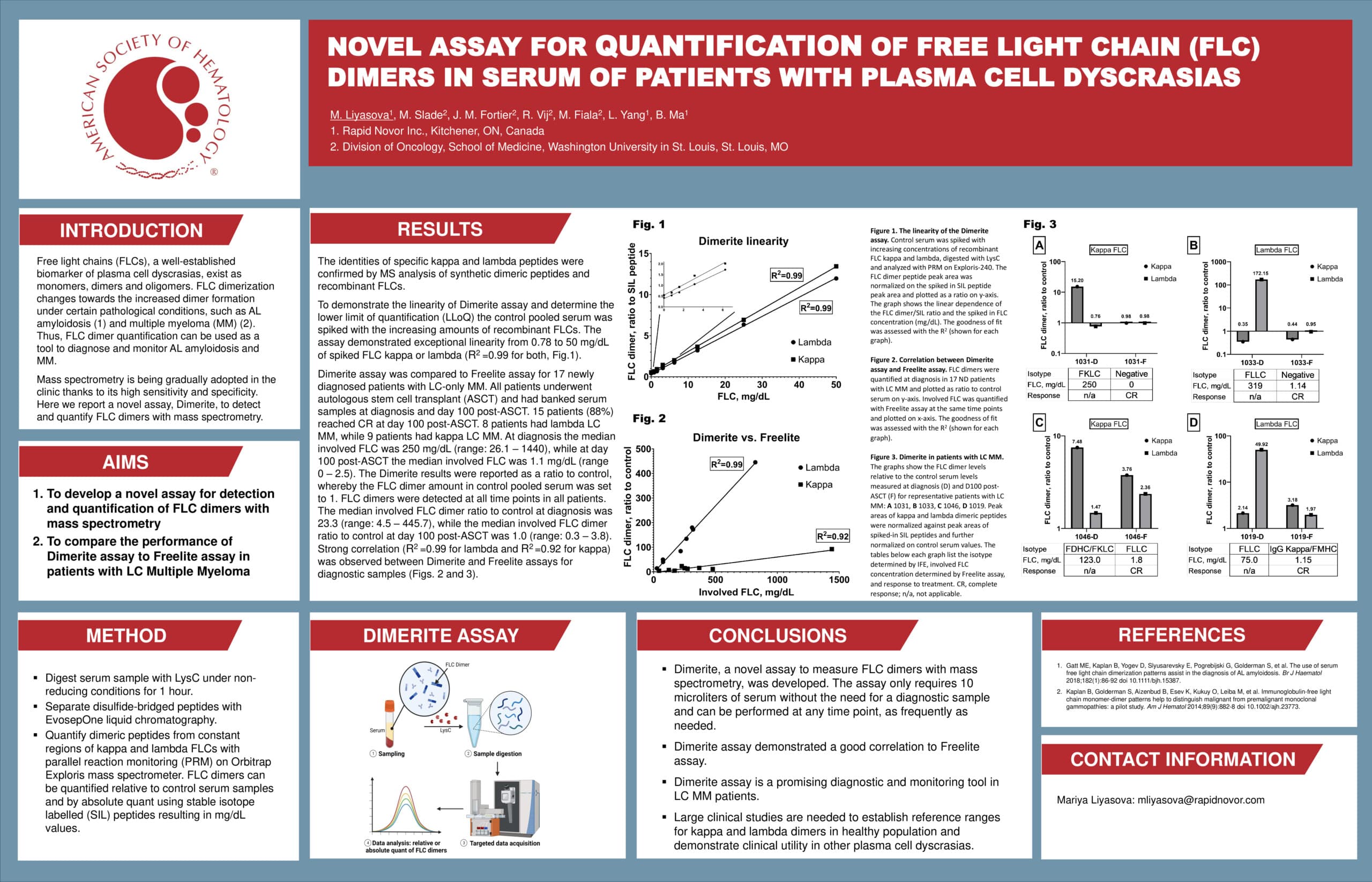
To demonstrate the linearity of Dimerite assay and determine the lower limit of quantification (LLoQ), the control pool serum was spiked with increasing amounts of recombinant FLCs.
Dimerite results were reported as a ratio to control, whereby the FLC dimer amount in control pooled serum was set to 1.
Key Takeaways:
- Dimerite demonstrated exceptional linearity from 0.78 to 50 mg/dL of spiked FLC kappa or lambda (R2 = 0.99 for both)
- FLC dimers were detected at all time points in all 17 LC-only MM patients
Strong correlation was observed between Dimerite and Freelite assays for diagnostic samples (R2 = 0.99 for lambda and R2 = 0.92 for kappa)
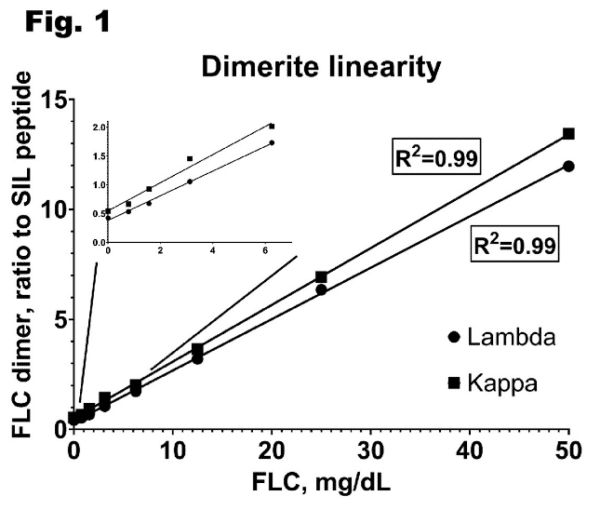
Fig 1. The linearity of the Dimerite assay
Fig 2. Correlation between Dimerite and Freelite assay
Conclusions
Dimerite is a novel mass spectrometry-based assay for the detection and quantification of Free Light Chain (FLC) dimers in the serum of patients with plasma cell dyscrasias. The assay showed excellent linearity and correlation with the standard Freelite assay, demonstrating its potential as a diagnostic and monitoring tool for conditions like light chain multiple myeloma (LC MM).
This technology is related to EasyM but is not part of the EasyM assay. Dimerite is still in development and further clinical studies are sought.
Click to enlarge poster.
Authors
Liyasova, M., Slade, M., Fortier, J., Vij, R., Fiala, M., Yang, L., & Ma, B. (2023). In Oral and Poster Abstracts, Session 803. Emerging Tools, Techniques and Artificial Intelligence in Hematology: Poster I. Presented at the American Society of Hematology Annual Meeting, December 9, 2023.
Contact Us.
We want to make an impact on myeloma.
We welcome discussion with academic investigators, pharmaceutical companies, patient groups and related service providers. Please reach out.
Talk to Our Scientists.
We Have Sequenced 5000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics