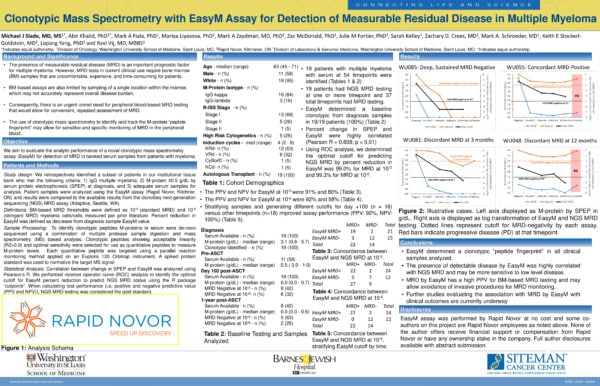
Introduction
The presence of minimal residual disease (MRD) is an important prognostic factor for multiple myeloma. However, MRD tests in current clinical use require bone marrow (BM) samples that are uncomfortable, expensive, and time-consuming for patients. BM-based assays are also limited by sampling of a single location within the marrow, which may not accurately represent the overall disease burden. Consequently, there is an urgent unmet need for peripheral blood-based MRD testing that would allow for convenient and frequently repeatable assessment of MRD.
Study Summary
This study assessed the use of EasyM, a highly sensitive clonotypic mass spectrometry (MS) MRD assay (Rapid Novor, Kitchener, Canada).
19 patients were retrospectively identified from the institutional tissue bank who met the following criteria:
- IgG multiple myeloma
- M-protein ≥0.5 g/dL by serum protein electrophoresis (SPEP) at diagnosis
- adequate samples for analysis.
EasyM results were subsequently compared to SPEP and, when available, next generation sequencing MRD testing with the clonoSeq assay (Adaptive, Seattle, WA).
Key Takeaways:
- In all 19 patients, a clonotypic peptide signature was identified from the baseline diagnostic samples
- Percent change in EasyM and SPEP were well correlated (Pearson R = 0.808; p < 0.01)
- Exploratory analysis was conducted at day 100 post-ASCT to assess EasyM MRD vs NGS MRD at 10-5. To account for the delayed clearance of IgG, an optimal cutoff of 99% percent reduction by EasyM at day 100 was determined through receiver operator curve (ROC) analysis
- The PPV and NPV for EasyM at 10-5 were 91% and 80%. The PPV and NPV for EasyM at 10-6 were 92% and 58%
- One patient had undetectable EasyM at day 100 post-ASCT, received 4 months of post-ASCT lenalidomide, but remains in CR over 3 years after transplant
- Two patients achieved undetectable EasyM at 9 and 55 months post-ASCT and remained in CR at 52 and 64 months
- 79% of samples that were MRD negative at 10-5 by clonoSeq had detectable M-protein by clonotypic MS
Conclusions
Our study provides initial data regarding clonotypic MS with the EasyM assay for peripheral blood MRD testing. We demonstrated an excellent correlation between percent reduction in M-protein by SPEP and EasyM. The presence of detectable disease by EasyM was highly correlated with NGS MRD and may be more sensitive to low level disease. MRD by EasyM has a high PPV for BM-based MRD testing and may allow for alternatives to invasive procedures for MRD monitoring.
Authors
Michael J Slade, Abir Khalid, Mark A Fiala, Mariya Liyasova, Mark A Zaydman, , Zac McDonald, Julie M Fortier, Sarah Kelley, Zachary D. Crees, Mark A. Schroeder, Keith E Stockerl-Goldstein, Liqiang Yang and Ravi Vij. American Society of Hematology Annual Meeting and Expo 2023, Poster 1908, Session 652
Contact Us.
We want to make an impact on myeloma.
We welcome discussion with academic investigators, pharmaceutical companies, patient groups and related service providers. Please reach out.
Talk to Our Scientists.
We Have Sequenced 5000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics