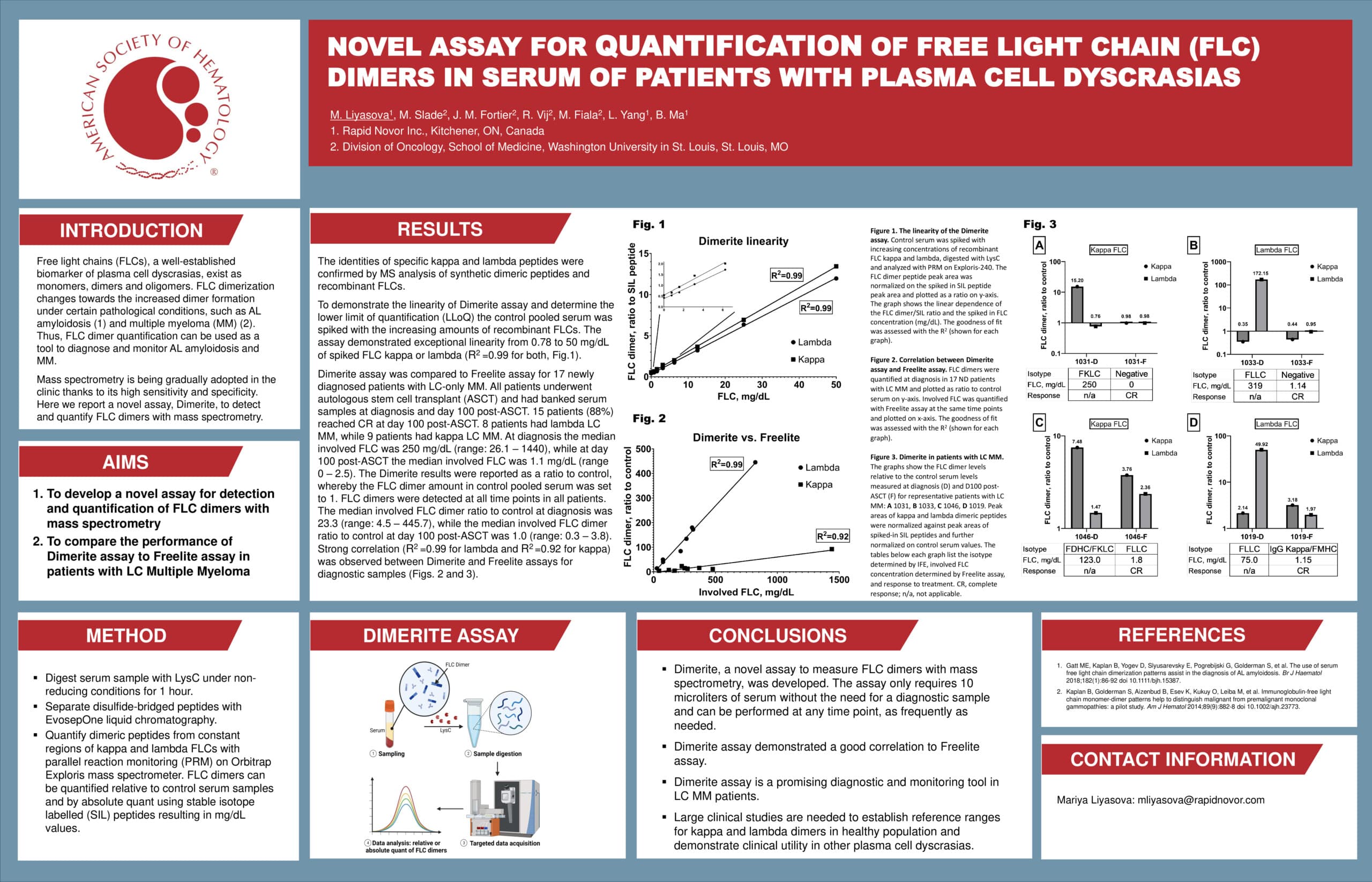
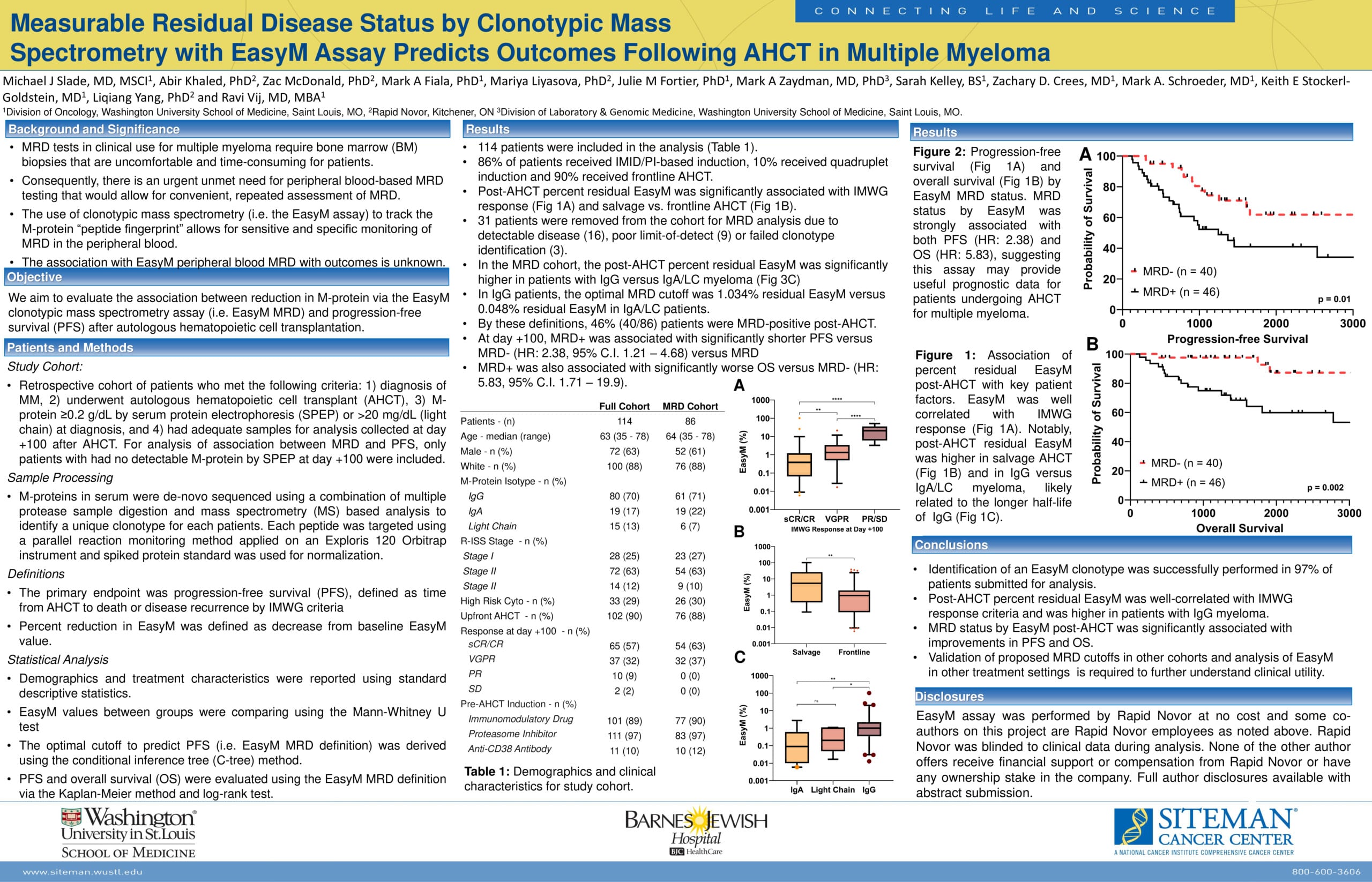
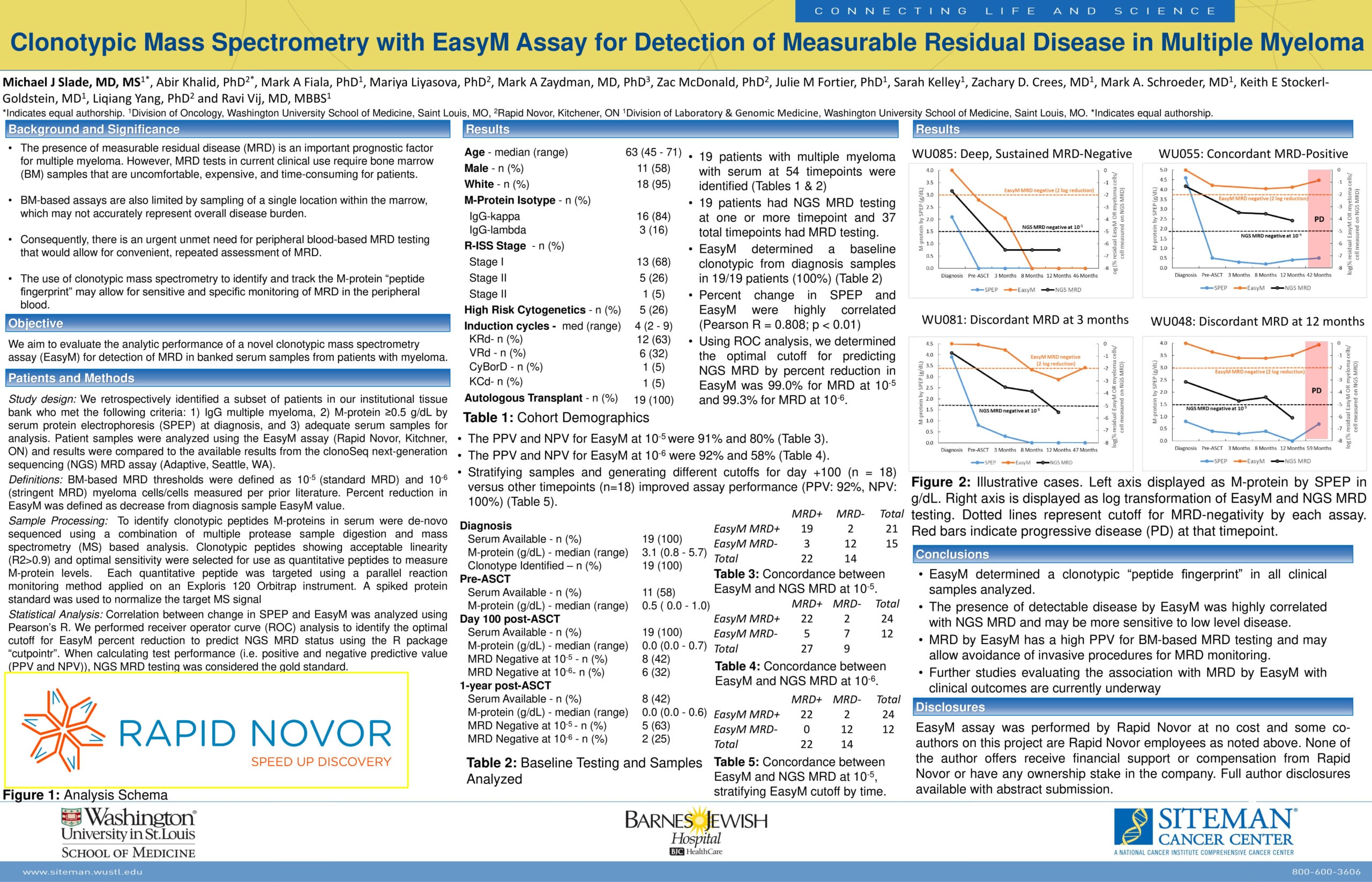
Comparison of EasyM Clonotypic Mass Spectrometry and EuroFlow MRD in Multiple Myeloma
Introduction Here, we summarize a retrospective comparison of EasyM and Euroflow NGF for MRD in newly diagnosed multiple myeloma patients. Minimal residual disease (MRD) negativity is a critical measure of therapeutic response in multiple myeloma (MM) and is strongly associated with improved progression-free and overall survival. The IMWG (International Myeloma Working Group) [...]