Minimal Residual Disease is defined as the small number of cancer cells that can remain in the body during or after treatment.
These residual cells are often not detectable by conventional testing methods and can lead to disease relapse in the future. This makes MRD an important consideration in the management of multiple myeloma (MM).
Why is measuring MRD so important?
Evaluating Treatment
Response
Response
Advancements in treatment options have enabled more myeloma patients to achieve a complete response (CR). However, relapses can often still occur.
Monitoring for the presence or absence of residual myeloma cells (MRD positivity or negativity) can help doctors determine the likelihood of relapse and inform whether further cancer treatment is necessary.
A Useful
Prognostic Tool
Prognostic Tool
Minimal residual disease negativity has been linked to better clinical outcomes.
Studies have shown that patients who achieved and sustained MRD negativity at a deeper level had the greatest improvement in progression-free survival and overall survival.
MRD-Guided
Treatment Decisions
Treatment Decisions
Doctors are actively exploring using MRD status to guide treatment decisions in several prospective trials. These include the MASTER trial, MRD2STOP trial and the ongoing DRAMMATIC trial.
New blood-based techniques, such as mass spectrometry, can provide a convenient and non-invasive alternative to standard MRD tests that currently rely on bone marrow samples.
Current Methods for MRD Testing
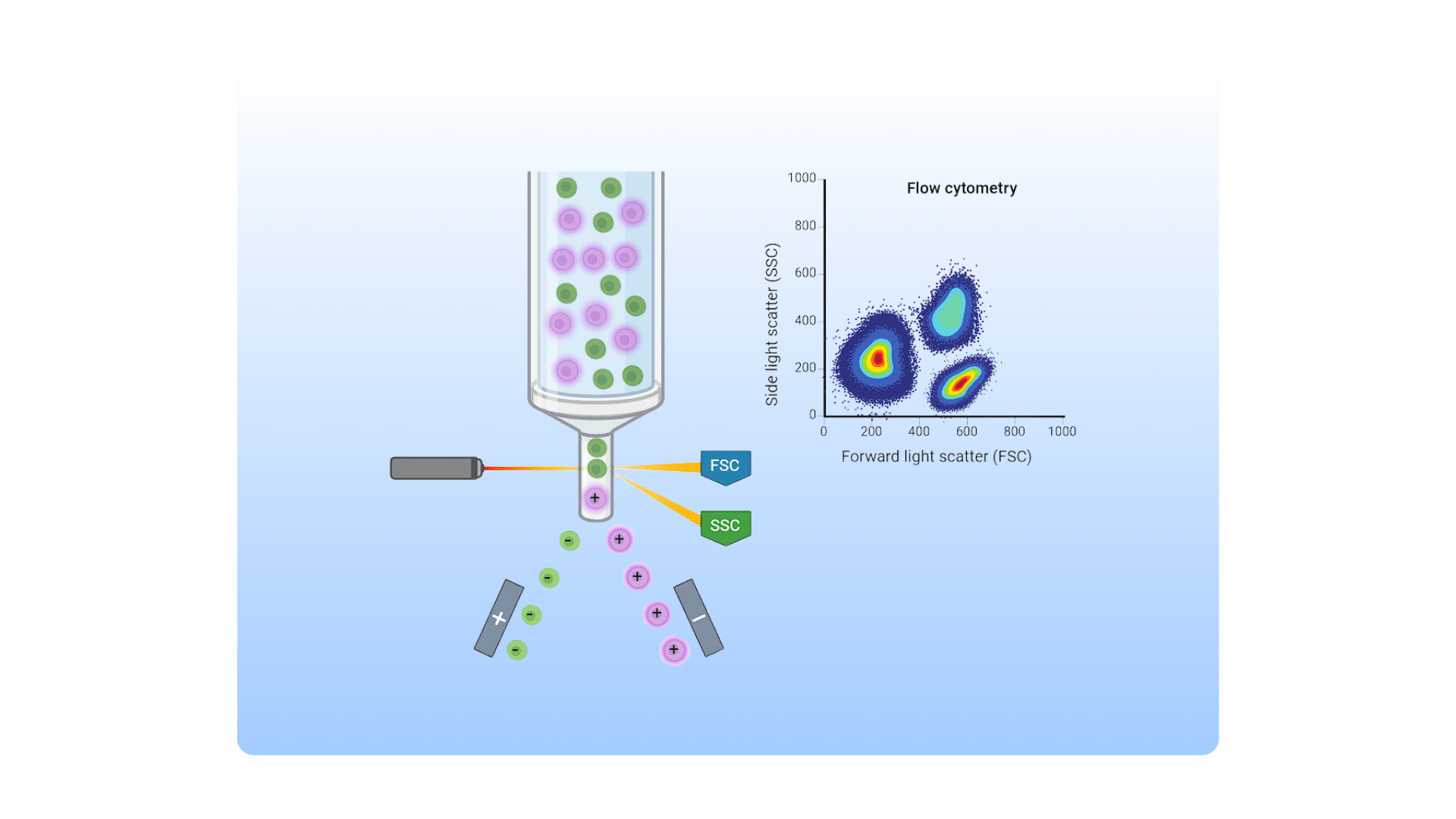
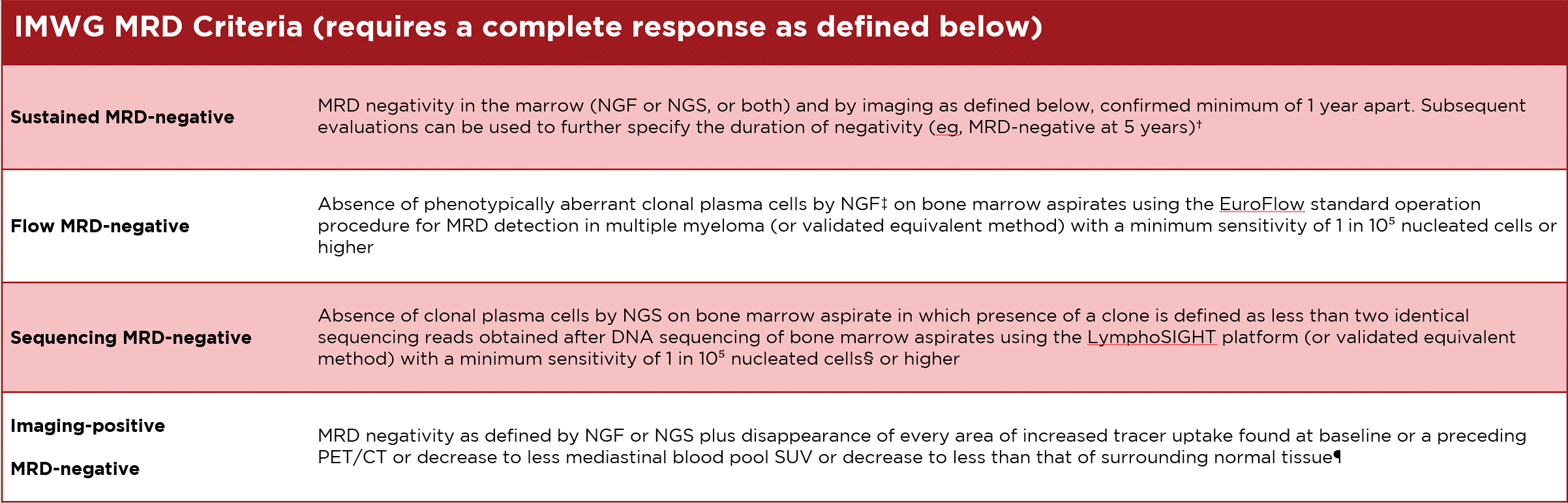
The International Myeloma Working Group (IMWG) defines MRD negativity as the absence of myeloma cells in a sample of 100,000 cells either by next generation flow cytometry (NGF) or next generation sequencing (NGS). Both methods require samples collected from painful and invasive bone marrow aspiration or biopsy. This puts limits on how often they can be used for routine follow ups.
Obtaining quality bone marrow samples can be challenging due to the patchy nature of myeloma. Poor sample quality or hemodilution can underestimate the number of residual cells or give false negative test results. Clinicians may also use imaging tests, such as PET scanning or MRI, in addition to NGF or NGS to identify bone lesions or evidence of extramedullary disease (EMD).
- Uses distinctive cell surface markers to identify and track myeloma cells
- Can identify one cancer cell among 100,000 normal cells (sensitivity of 10-5)
- Offers the highest sensitivity by identifying unique DNA sequences to track myeloma cells
- Can detect as few as one cancer cell in 1,000,000 normal cells (sensitivity of 10-6)
†Sustained MRD negativity when reported should also annotate the method used (eg, sustained flow MRD-negative, sustained sequencing MRD-negative).
‡Bone marrow MFC should follow NGF guidelines. The reference NGF method is an eight-colour two-tube approach, which has been extensively validated. The two-tube approach improves reliability, consistency, and sensitivity because of the acquisition of a greater number of cells. The eight-colour technology is widely available globally and the NGF method has already been adopted in many flow laboratories worldwide. The complete eight-colour method is most efficient using a lyophilised mixture of antibodies which reduces errors, time, and costs. 5 million cells should be assessed. The FCM method employed should have a sensitivity of detection of at least 1 in 10⁵ plasma cells.
§DNA sequencing assay on bone marrow aspirate should use a validated assay such as LymphoSIGHT (Sequenta).
¶Criteria used by Zamagni and colleagues,85 and expert panel (IMPetUs; Italian Myeloma criteria for PET Use)
Introducing EasyM™: MRD Blood Test for Multiple Myeloma
EasyM is a groundbreaking blood test for multiple myeloma MRD monitoring.
EasyM uses the ultrasensitive clonotypic peptides mass spectrometry (MS) method to measure the amount of abnormal monoclonal antibody proteins (M-spike) made by your myeloma cells in the blood.