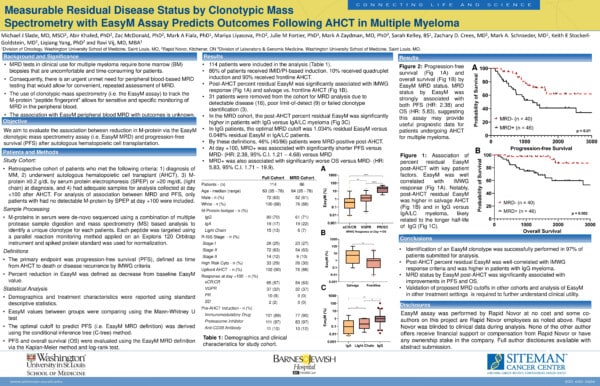
Introduction
Bone marrow (BM)-based minimal residual disease (MRD) has been established as one of the most important dynamic prognostic markers in multiple myeloma (MM). However, the necessity of bone marrow biopsies limits the feasibility of frequent MRD monitoring. Consequently, there is an urgent unmet need for peripheral blood-based MRD testing for serial monitoring of disease response to allow tailored therapy for patients achieving deep remissions.
Quantification of myeloma (M)-protein using mass spectrometry (MS) is a promising approach for peripheral blood-based MRD monitoring due to its exceptional analytical sensitivity. Prior studies have examined the use of MALDI-TOF-based methods, which relies on the mass distribution of intact immunoglobulins light chains and is approximately 1-log more sensitive than traditional testing. However, recent advances in MS technology have allowed the determination and tracking of a “clonotypic” MS signature in peripheral blood which is highly sensitive and patient-specific (Liyasova et al, CCR 2021).
Study Summary
A large retrospective analysis of 86 patients enrolled in our institutional MM tissue bank was performed to determine the association of clinical outcomes with clonotypic MS-based MRD testing (EasyM, Rapid Novor, Kitchener, ON).
Patients were selected from our institutional tissue bank who met the following criteria:
- Diagnosis of MM,
- Underwent autologous hematopoietic cell transplant (AHCT),
- M-protein ≥0.2 g/dL by serum protein electrophoresis (SPEP) or >20 mg/dL (light chain) at diagnosis,
- No detectable M-protein by SPEP at day +100
- Samples for analysis collected at day +100 after AHCT.
Percent residual EasyM at day 100, in respect to baseline EasyM, was used as the independent variable to predict Progression-free survival (PFS). EasyM MRD+/- status was defined as being above or below the optimal MRD cutoff levels at day 100, respectively.
Key Takeaways:
- Identification of an EasyM clonotype was successfully performed in 97% of patients submitted for analysis
- In the MRD cohort, the day 100 post-AHCT percent residual EasyM was significantly higher in patients with IgG versus igA/LC myeloma, likely due to the differing half-lives of these molecules
- In IgG patients, the optimal MRD cutoff at day 100 post AHCT was 1.034% residual EasyM as compared to 0.048% residual EasyM for IgA/LC patients
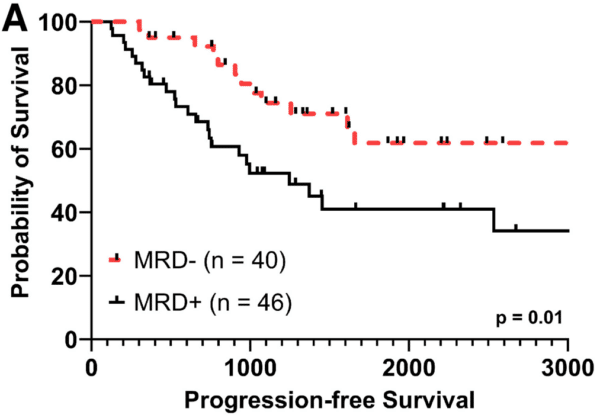
Fig 1A. At day 100, EasyM MRD+ was associated with significantly shorter PFS versus Easym MRD- (HR: 2.38, 95% C.I. 1.21 – 4.68)
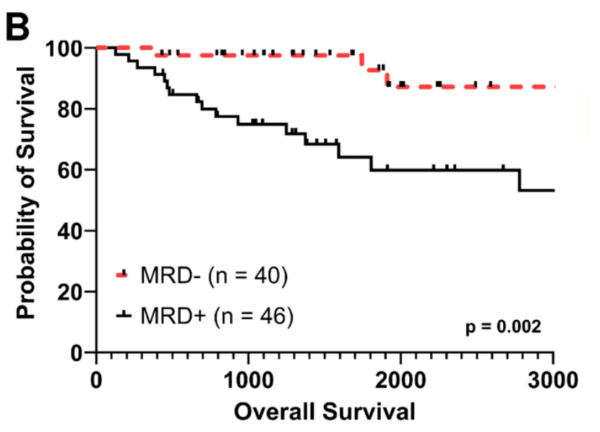
Fig 1B. EasyM MRD+ was also associated with significantly worse OS versus EasyM MRD- (HR: 5.83, 95% C.I. 1.71 – 19.9)
Conclusions
Our study is the first, to our knowledge, to demonstrate the clinical utility of peripheral blood-based clonotypic mass spectrometry to predict outcomes for patients with MM. MRD status by EasyM post-AHCT was significantly associated with improvements in PFS and OS. Notably, our results suggest that different cutoffs should be employed for IgG and IgA/LC myeloma if drawn at day +100 after transplant. This is possibly due to the differing half-lives of these molecules.
Click to enlarge poster.
Authors
Michael Slade, Abir Khaled, Mark Fiala, Zac McDonald, Mariya Liyasova, Julie Fortier, Mark Zaydman, Sarah Kelley, Zachary David Crees, Mark A. Schroeder, Keith Stockerl-Goldstein, Liqiang Yang, Ravi Vij
Contact Us.
We want to make an impact on myeloma.
We welcome discussion with academic investigators, pharmaceutical companies, patient groups and related service providers. Please reach out.
Talk to Our Scientists.
We Have Sequenced 5000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics