Precision MRD Blood Test for Multiple Myeloma.
EasyM™ is an ultrasensitive mass spectrometry-based blood test for Multiple Myeloma (MM) minimal residual disease (MRD) monitoring.
Precision MRD Blood Test for Multiple Myeloma
Advances in multiple myeloma treatments have led to improved survival in patients with multiple myeloma. Achieving minimal residual disease negativity is strongly linked to longer Progression-Free Survival (PFS) and Overall Survival (OS).1,2
The 2016 update to the IMWG response criteria added new definitions for MRD negativity, and in 2024, the FDA recognized MRD as a surrogate endpoint in clinical trials. These changes have increased MRD’s importance in clinical practice and underscored the need for better MRD monitoring tools.
Highly sensitive bone marrow tests for multiple myeloma like next generation sequencing (NGS) and next generation flow cytometry (NGF) are commonly used to assess MRD after autologous stem cell transplant (ASCT) or during maintenance therapy. However, because they require bone marrow aspirations, frequent use in practice is limited.3
EasyM™: A Simpler Way to Monitor MRD in MM
EasyM is a groundbreaking blood test for MRD monitoring in multiple myeloma.
M-Protein in multiple myeloma is a well established biomarker. EasyM uses ultrasensitive clonotypic peptides mass spectrometry to measure M-protein levels in the blood.
Powered by Rapid Novor’s REmAb platform and proprietary proteomics workflow, it quickly and accurately sequences M-proteins from a simple serum sample.
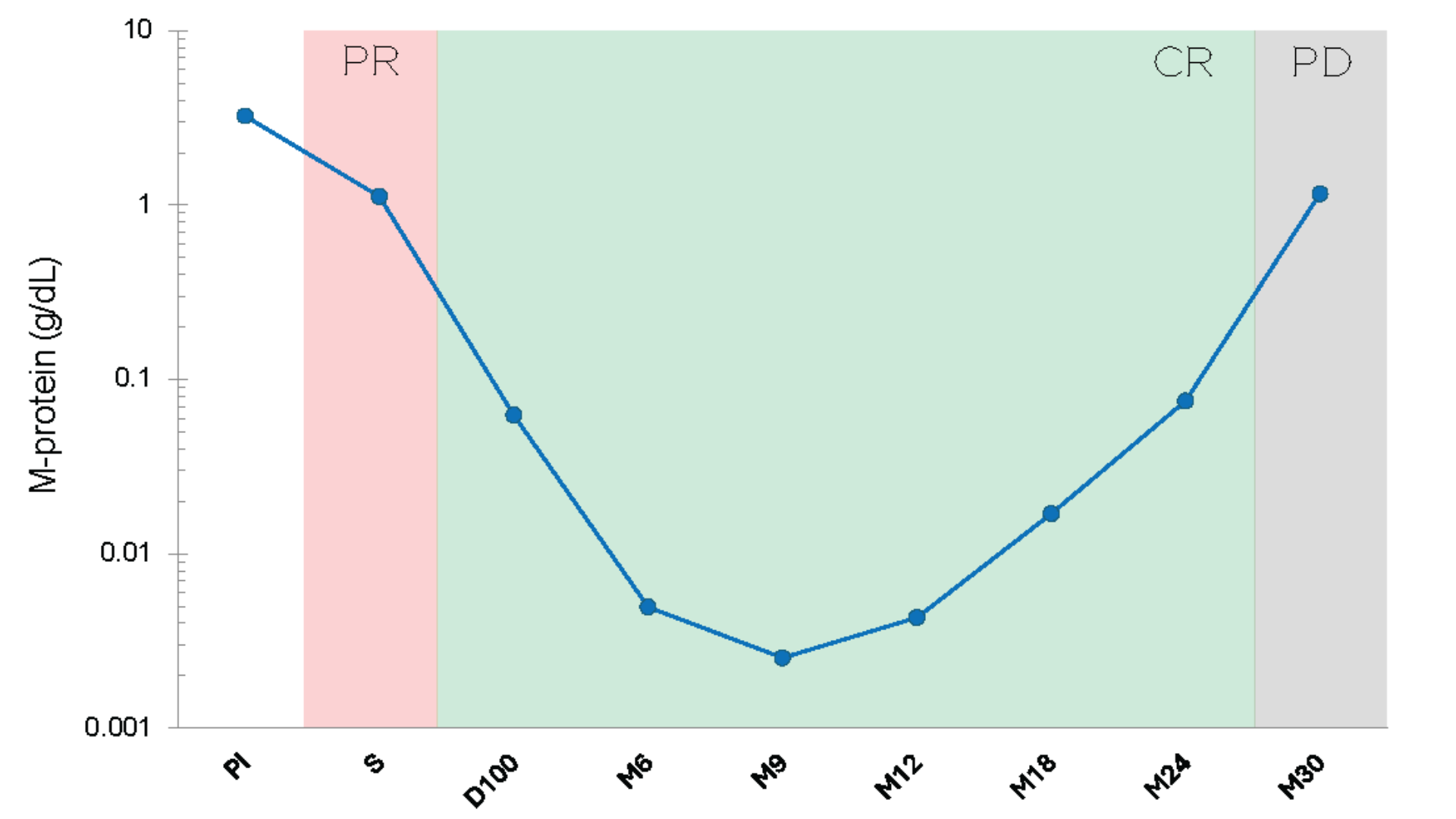
Follow the M-protein through the patient's deepest response.
Detect myeloma relapse earlier.
Liyasova M, et al. Clin Cancer Res. 2021; 27(18):5028-5037
Explore our publications and posters.
EasyM is currently certified for clinical use under CLIA in all US states with the exception of NY. EasyM has not been cleared by the FDA and is not yet covered by commercial health insurance or Medicare/Medicaid.
For Clinical Collaborations
The multiple myeloma community benefits from exploration of MRD techniques using real-world clinical data. Contact us to explore inclusion of EasyM in your previous or upcoming clinical trials.
2025 Conferences.
Come meet us at one of the many myeloma conferences we’ll be attending or exhibiting at this year:
References
- Landgren, O. et al. Bone Marrow Transplant. (2016) 51:1565– 8.
- Kaddoura, M. et al. Am J Hematol. 2022; 97(3): 267- 273
- Kumar, S. et al. Lancet Oncol. 2016;17(8):e328-e346
- Slade M, et al. Blood 2022; 140 (Supplement 1): 4376–4377.
- Liyasova M, et al. Clin Cancer Res. 2021; 27(18):5028-5037