Multiple Myeloma Blood Test for MRD Monitoring.
What is EasyM™ ?
EasyM™ is an ultrasensitive, non-invasive blood test to monitor minimal residual disease (MRD) in patients with multiple myeloma (MM).
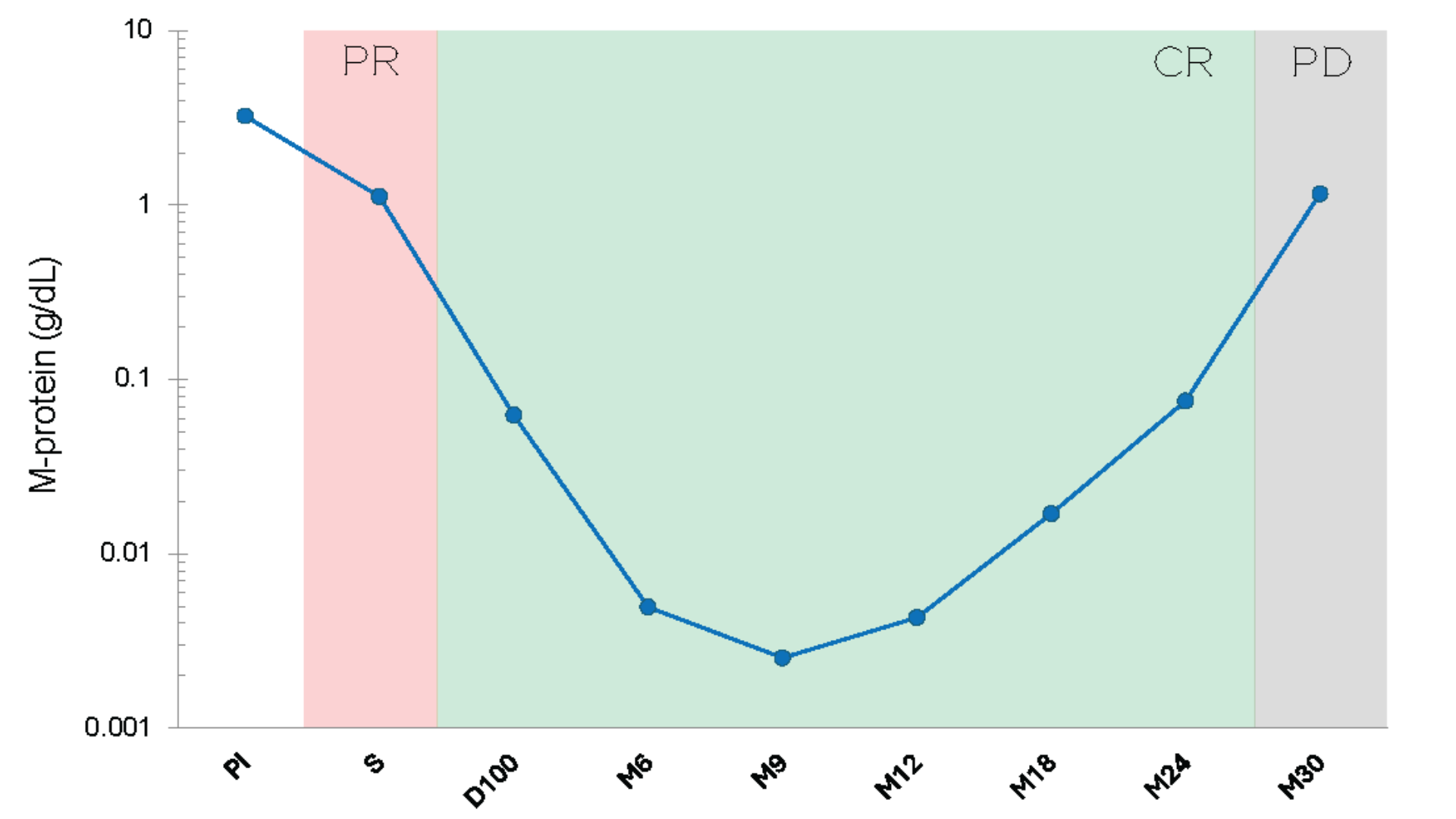
EasyM uses clonotypic peptides mass spectrometry to track M-protein (monoclonal protein) in peripheral blood to extremely low levels, offering patients a easier and non-invasive alternative to track MRD during complete response (CR).
*Preliminary results based on interim data, are subject to change.
Track M-Protein into CR
M-Protein Doubling Precedes Relapse3
Additional Information
I am a Clinician.
Learn about the benefits of the EasyM clonotypic peptides mass spectrometry test for MRD monitoring in multiple myeloma.
See how it can be useful in your clinical practice.
I am a Patient.
Explore our vision to bring convenient, blood-based MRD monitoring to patients living with multiple myeloma.
I am from Industry.
Consider including EasyM™ for improved disease monitoring in MRD-guided clinical trials.
Include patients with extramedullary disease and obtain additional timepoints with blood-based testing.
2025 Conferences.
Come meet us at one of the many myeloma conferences we’ll be attending or exhibiting at this year:
Sign up to follow our progress with bringing EasyM™ to the Myeloma community.
References
1. Slade M, et al. Blood 2022; 140 (Supplement 1): 4376–4377.
2. Zhao J, et al. Haematologica 2024 doi: 10.3324/haematol.2024.285933
3. Liyasova M, et al. Clin Cancer Res. 2021 Sep 15;27(18):5028-5037.